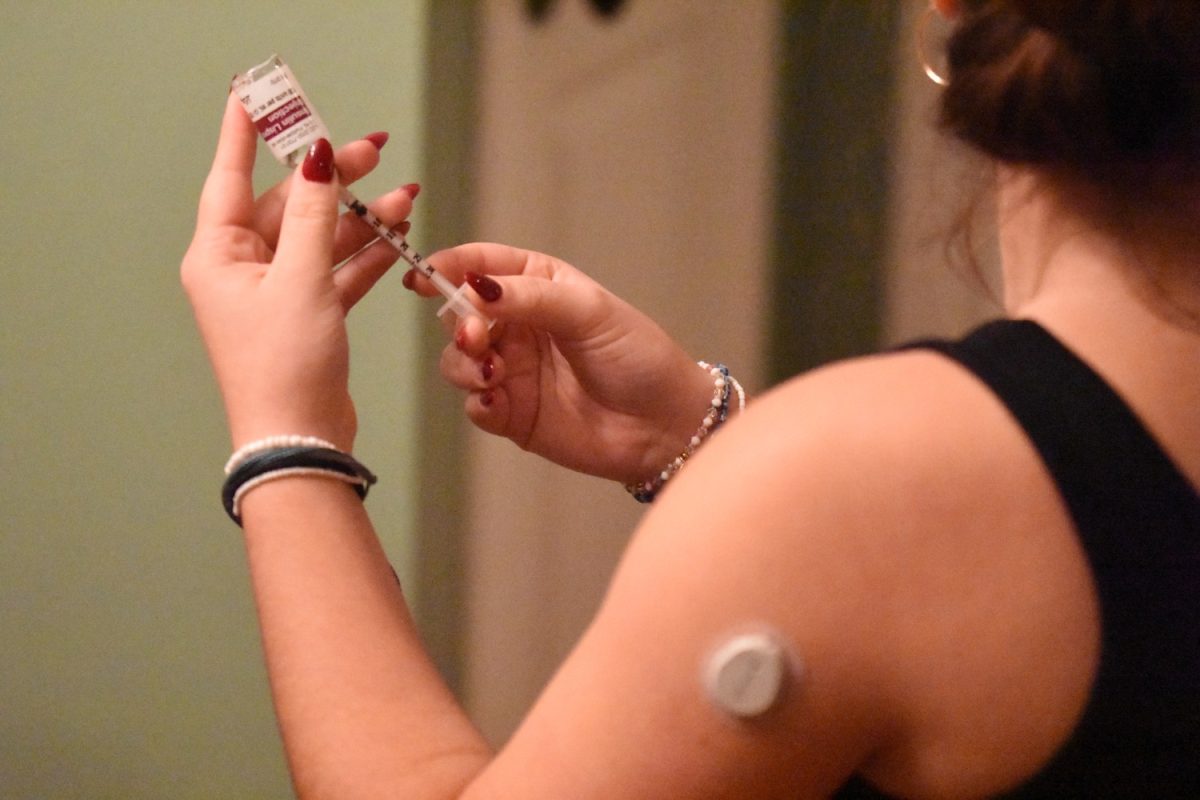From the moment the coronavirus was declared a pandemic by the World Health Organization (WHO), the race to find a vaccine for COVID-19 has ensued. Many vaccine trials have taken place, but no vaccine has been promising.
However, on Nov. 9, Pfizer, in collaboration with BioNTech, announced that their vaccine was more than 90% effective in preventing COVID-19 in the test participants.
“Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19,” said Dr. Albert Bourla, Pfizer Chairman and CEO.
Pfizer and BioNTech announced that their mRNA-based vaccine could combat COVID-19 in participants during the Phase 3 clinical study.
The vaccine has given a boost of confidence to many that an end to the pandemic is close, as BioNTech CEO Ugur Sahin is hopeful that life will return to normal by next winter.
“This vaccine is a huge breakthrough, not only for the pandemic but for scientists and vaccine creators as well. We have never created a vaccine for a global pandemic this quickly with the hopes of it being this effective. This vaccine could result in a dramatic reduction of the spread,” said Nicholas Voong, a junior at Carlmont.
Despite the positive trial results, the vaccine awaits approval from regulators. According to CNN, Americans should not be hoping for any authorization from the US Food and Drug Administration (FDA) before the last half of December.
Given the uncertainty of the vaccine, the Centers for Disease Control (CDC) produced a vaccination plan “interim playbook” that Alex Azar, US Health and Human Services secretary, aims to follow. Within the plan, essential workers and those at higher risk will be the first to receive the vaccine, though the plan is not entirely finalized.
Aside from the question of who gets the vaccine first, the federal government believes the earliest the general public would receive the vaccine will likely be in the spring of 2021.
“I believe that it is right for the medical workers and front line workers to get the vaccine first because they are the most susceptible to getting COVID-19. Though spring is a couple of months away, it is good we have a vaccine that has possibilities of being this effective,” said junior Nadine Lahlouh.
Pfizer has spotlighted the future distribution of its COVID-19 vaccine, as it presents a potential hurdle: It needs to be stored at around negative 70 degrees Celsius (94 degrees Fahrenheit).
This cold temperature is uncommon for a vaccine. According to NPR, it provides a challenge to some distributors who are scrambling to find dry ice or other mechanisms to keep the vaccine cold because negative 70 degrees Celcius is colder than a refrigerator.
“We have developed packaging and storage innovations to be fit for purpose for the range of locations where we believe vaccinations will take place. We have already initiated the development of innovative cold-chain solutions and distribution logistics to facilitate vaccine supply,” said Steven Danehy, Pfizer spokesperson.
Given the distribution plans, the next step for the vaccine is the determination of side effects; according to NBC News, scientists predict that the shots will cause enervating flu-like side effects such as a sore arm, muscle aches, or a fever that could last for a few days.
“The biggest tragedy would be if we have a safe and effective vaccine that people are hesitant to get,” said Dr. Preeti Malani, the Chief Health Officer and a professor of medicine at the University of Michigan in Ann Arbor, in an interview with NBC News.
On Nov. 16, Moderna, an American biotechnology company, said that their vaccine is 94.5% effective against COVID-19, according to early data.
“We are now given two vaccines with astonishing high effectiveness rates. This is extremely exciting to see. The next move is to test them over the next couple of weeks to determine their potential side effects,” said Teasha Zhou, a junior at Carlmont.
The company says its vaccine did not have any known severe side effects, and according to CNN, a just small percentage of those who received it experienced symptoms such as body aches and headaches.
While the two vaccines are similar in their mechanics, using mRNA to fight the coronavirus, the Moderna vaccine has some advantages over the Pfizer-BioNtech vaccine.
The Moderna vaccine can be kept at negative 20 degrees Celsius (negative 4 degrees Fahrenheit) compared to the Pfizer-BioNtech vaccine that must be stored at negative 70 degrees Celsius. Because of it’s lower storage temperature, the Moderna vaccine can be kept in freezers that are already available in many hospitals and pharmacies, while the Pfizer-BioNtech vaccine requires dry ice or a colder freezer for storage.
Further, the Moderna vaccine can be kept for 30 days in the refrigerator, while the Pfizer-BioNtech vaccine only can last five days in a refrigerator at a negative 70 degrees Celsius.
“It is a race for the vaccine. The only thing left to do is test the side effects, get it approved, and then start shipping it out for everyone. We are very close to the end of this pandemic,” Zhou said.